

Number of orbitals in designated subshell The names of the orbitals are named after the subshells they are found in: For example, if l=1 and m l can have values -1, 0, or +1, the value of 2 l+1 will be three and there will be three different orbitals. This equation will not give you the value of m l, but the number of possible values that ml can take on in a particular orbital. A helpful equation to determine the number of orbitals in a subshell is 2 l +1. The number of orbitals in a subshell is equivalent to the number of values the magnetic quantum number ml takes on. For example, 3p refers to the third principal quantum number (n=3) and the p subshell (l=1). We can designate a principal quantum number, n, and a certain subshell by combining the value of n and the name of the subshell (which can be found using l). Principal shell with n = 3 has one s subshell, one p subshell, and one d subshell (l = 0, 1, 2).Principal shell with n = 2 has one s subshell and one p subshell ( l = 0, 1).Principal shell with n = 1 has one s subshell ( l = 0).The value of l determines the name of the subshell: Name of Subshell To identify what type of possible subshells n has, these subshells have been assigned letter names. Principal shell with n = 3 has three subshells.Principal shell with n = 2 has two subshells.Principal shell with n = 1 has one subshell.When n = 3, l= 0, 1, 2 ( l takes on three values and thus there are three possible subshells)Īfter looking at the examples above, we see that the value of n is equal to the number of subshells in a principal electronic shell:.When n = 2, l= 0, 1 ( l takes on two values and thus there are two possible subshells).When n = 1, l= 0 ( l takes on one value and thus there can only be one subshell).The number of values of the orbital angular number l can also be used to identify the number of subshells in a principal electron shell:
As the energy of the electron increases, so does the principal quantum number, e.g., n = 3 indicates the third principal shell, n = 4 indicates the fourth principal shell, and so on. Known as emission, electrons can also “emit” energy as they jump to lower principle shells, where n decreases by whole numbers. This is called absorption because the electron is “absorbing” photons, or energy. This explains why n can not be 0 or any negative integer, because there exists no atoms with zero or a negative amount of energy levels/principal shells. When an electron is in an excited state or it gains energy, it may jump to the second principle shell, where. The first principal shell is also called the ground state, or lowest energy state. n can be any positive integer starting at 1, as n=1 designates the first principal shell (the innermost shell). Because n describes the most probable distance of the electrons from the nucleus, the larger the number n is, the farther the electron is from the nucleus, the larger the size of the orbital, and the larger the atom is. The principal quantum number, n, designates the principal electron shell. The magnetic quantum number, m l, describes the energy levels in a subshell, and m s refers to the spin on the electron, which can either be up or down. It can also be used to determine the number of angular nodes.

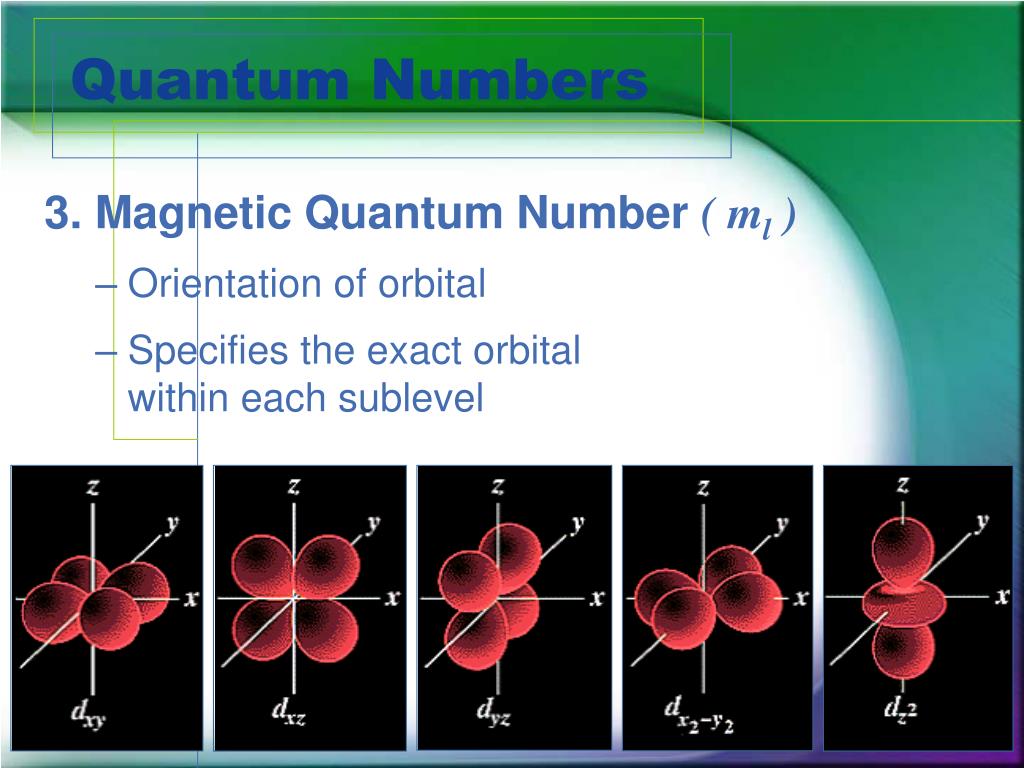
The number of subshells, or l, describes the shape of the orbital. In other words, it refers to the size of the orbital and the energy level an electron is placed in. The principal quantum number, n, describes the energy of an electron and the most probable distance of the electron from the nucleus. There are a total of four quantum numbers: the principal quantum number ( n), the orbital angular momentum quantum number ( l), the magnetic quantum number ( m l), and the electron spin quantum number ( m s). This means that they describe completely the characteristics of an electron in an atom, i.e., they describe each unique solution to the Schrödinger equation, or the wave function, of electrons in an atom. Quantum numbers designate specific shells, subshells, orbitals, and spins of electrons.


 0 kommentar(er)
0 kommentar(er)
